Medical Device Authority MDA is a statutory body under the Ministry of Health Malaysia which was established under the Medical Device Authority Act 2012 Act 738 to control regulate. Malaysia currently imports around 95 of the medical device for its consumption In Malaysia the medical device industry is a highly diversified industry that.
Peranti Perubatan Umum Medical Device Authority Mda
Medical device product registration in Malaysia is overseen by the Medical Device Authority MDA of the Ministry of Health Malaysia MoHM as stipulated under the Medical Device Act.
. This Guidance Document shall be read in conjunction with the current laws and. Regulations used in Malaysia which include. Malaysia Medical Devices Regulations.
The Act strives to ensure that medical devices in Malaysia are. To obtain market authorization in Malaysia you must. Medical Device Act of 2012 Chapter 2 10-14.
Medical Device Act 2012. KEMENTERIAN KESIHATAN MALAYSIA MEDICAL DEVICE SECTION 2 ACT 737 medical device means any instrument apparatus implement machine appliance implant in vitro reagent or calibrator software material or other similar or related article. For that purpose an.
Medical device registration in Malaysia is regulated by the Medical Device Authority MDA a federal statutory agency under the Ministry of Health MoH. This is the latest gazettement of the Medical Device Regulations pursuant to the Medical Device Act 2012 Act 737. Malaysia Medical Device Act.
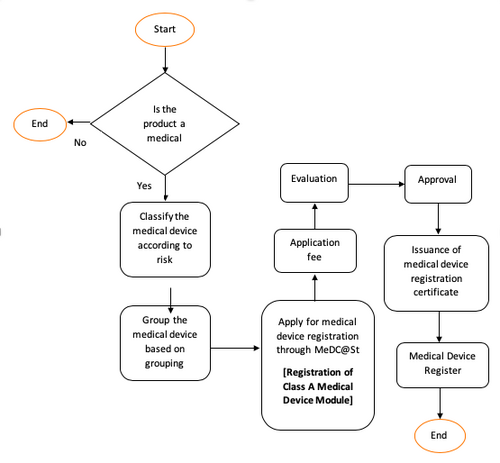
1 Section 51 of Medical Device Act 2012 Act 737 requires a medical device to be registered under the Act before it can be imported exported or placed in the market. The gazettement took effect on 3rd September 2019. New Guidance on Labelling Requirements for Medical Devices.
Yes medical devices do require registration before they can be sold in Malaysia. If there are violations of Act 737 and 2019 Regulation MDA may revoke and canceled the establishment license and certificate of the medical device. Malaysia offers one of Southeast Asias most robust and dynamic markets for foreign medical device manufacturers.
2 An application for registration of medical device shall be submitted to the Authority using Form MDA1 listed in the Register. A intended by the manufacturer to be used alone or in. 11 rows Medical Device Authority MDA Ministry of Health Malaysia Level 6 Prima 9 Prima Avenue II Block 3547 Persiaran APEC 63000 Cyberjaya Selangor MALAYSIA.
The main objective of the Medical Device Act is to protect public health and safety. Upper middle income Legal Legal framework. Enacted by the.
The Medical Device Regulations 2012 the subsidiary legislations under the Medical Device Act 2012 Act 737 has been approved by the. 27th September1971 Date of publication in the Gazette. Malaysia World Bank income group.
Medical device to be used for clinical investigation in Malaysia. Medical Device 7 laws OF MalaYsIa act 737 MedIcal devIce act 2012 An Act to regulate medical devices the industry and to provide for matters connected thereto. The Medical Device Authority MDA has prepared a guidance document on labelling requirements for medical.
Medical Device Exemption Order 2016. LAWS OF MALAYSIA ACT 50 MEDICAL ACT 1971 Incorporating latest amendment - PUA 172 2005 Date of Royal Assent. Two guidance documents aimed at supporting medical device manufacturers and Authorized Representatives comply with the Medical Device Act Act 737 and the regulations.
Device Act Act 737 and the regulations under it. Starting on July 1 2016 Malaysias Medical Device Act has made it mandatory for all foreign manufacturers to.
How To Apply For Establishment Licence Medical Device Authority Mda
Flow Chart The Official Portal Of Intellectual Property Corporation Of Malaysia Flow Chart Patent Registration Trips Agreement
Sample Temporary Guardianship Form Download Documents Pdf Instructions Forparental Guardian Approval Forminor Guardianship Printable Letter Templates Lettering
General Medical Device Medical Device Authority Mda
Medical Device Registration In Malaysia
Top 5 Environmental Stats Industrial Water Pollution Infographic Intelex Water Waterquality Waterpollution Pol Water Pollution Pollution Infographic
Testing And Compliance Verdict Medical Devices
Children With Disabilities In Malaysia The Big Picture What To Write About Writing Classes Unicef
General Medical Device Medical Device Authority Mda
Butterfly Iq Ultrasound Imaging System Medical Device Network
Pharmaboardroom Preclinical Clinical Trial Requirements Malaysia
Butterfly Iq Ultrasound Imaging System Medical Device Network
General Medical Device Medical Device Authority Mda

